In this article we will follow the chemical reactions during the manufacturing procedure of Portland cement and see how the ingredients interact to produce the product we all love, cement.
Manufacturing of Portland cement
The primary reactions during the calcination process are listed as below:The decomposition of clay happens at a temperature around 600◦C:
(b) Limestone (CaCO3) is mainly providing calcium (CaO) and is decomposed at 1000◦C:
(c) Iron ore and bauxite provide additional aluminum and iron oxide (Fe2O3), which help the
formation of calcium silicates at low temperature. They are incorporated into row mix.
formation of calcium silicates at low temperature. They are incorporated into row mix.
(d) There are different temperature zones in a rotary kiln. At various temperatures between
1000 and 1450◦C, different chemical compounds are formed. The initial formation of C2S occurs at a temperature of around 1200◦C. C3S is formed around 1400◦C.
(e) The final product from the rotary kiln is called clinker. Pulverizing the clinker into small
sizes (<75 µm) with addition of 3–5% gypsum or calcium sulf produces the Portland
cement. Gypsum added is to control fast setting caused by 3CaO · Al2O3.
sizes (<75 µm) with addition of 3–5% gypsum or calcium sulf produces the Portland
cement. Gypsum added is to control fast setting caused by 3CaO · Al2O3.
The majority of cement particle sizes are from 2 to 50 µm. Plots of typical particle size
distribution data analysis are given in the Chemical composition section below
Chemical composition
The majority of cement particle sizes are from 2 to 50 µm. Plots of typical particle size
distribution data analysis are given below
distribution data analysis are given below
Typical particle size distributions of Portland cement
Major compounds: The major compounds of ordinary Portland cement are listed in the table below. It should be indicated that C3S and C2S occupy 68 to 75% of Portland cement. Since the primary constituents of Portland cement are calcium silicates, we can define Portland cement as a material that combines CaO and SiO2 in such a proportion that the resulting calcium silicate will react with water at room temperature and normal pressure.
Major compounds of ordinary Portland cement
Minor components: The most important minor components of cement are gypsum, MgO, and alkali sulfates. Gypsum (2CaSO4 · 2H2O) is added in the last procedure of grinding the clinker to produce Portland cement. The reason for adding gypsum cement is to avoid the flash setting caused by fast reaction of C3A, because it can react with C3A and form a hydration product called ettringite on the surface of C3A to prevent further reaction of C3A as a barrier. The normal percentage of gypsum added cement is about 4–5%. Only when gypsum is more than 3% in a Portland cement, can the formation of ettringite be guaranteed. When the percentage of gypsum is between 1 and 3%, both ettringite and monosulfoaluminate will be formed. When the percentage of gypsum is lessthan 1%, only monosulfoaluminate will be formed. Alkalies (MgO, Na2O, and K2O) can increase the pH value of concrete up to 13.5, which is good for reinforcing steel protection. However, a high alkaline environment can also cause some durability problems, such as alkali aggregate reaction and leaching.
To read more about the concrete and its life-cycle, you can read this article



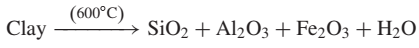




Comments
Post a Comment